Metallic bonding: an explanation
Metal atoms are held together by strong metallic bonds.
These bonds are produced by the negative free electrons which are strongly attracted to the positive nuclei and hold the atoms together in a giant metallic structure. Because the atoms are held together so strongly, a great deal of energy is needed to break the metallic bonds. As a result, metals have high melting and boiling points.
Instead of orbiting a particular nucleus, the electrons from the outer shell of every atom within the metal are shared amongst many other atoms. Together, they form a sea of delocalised electrons.
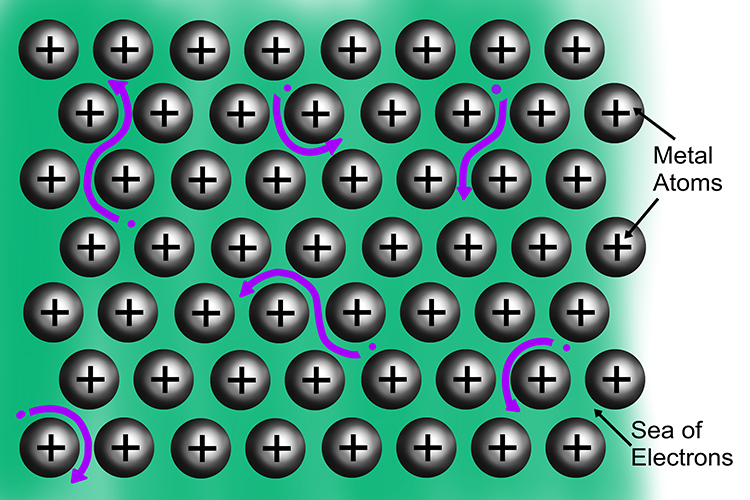
Within the ‘sea’, the electrons are free to move and drift. They are able to carry energy as they move about, making metals excellent conductors of heat and electricity.





