Properties of ionic substances
Ionic substances have high melting points
Ionic substances have very high melting points

Have you ever tried to melt salt? Its melting point is an incredible 801°C!
All ionic compounds have solid crystal structures
All ionic substances exist as solid crystal structures.

Think of salt as being a big crystal structure.
Ionic substances dissolve in water
Ionic substances often dissolve in water.

Salt dissolves in water.
Ionic compounds conduct electricity in solution
When salt dissolves in water, the sodium and chlorine dissociate from each other and this allows the solution to conduct electricity. (Note: in chemistry, the word is not 'disassociate')
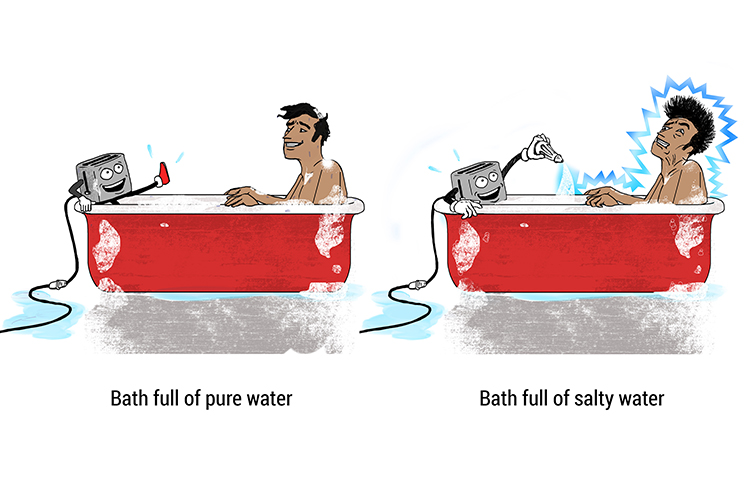
Pure water does not conduct electricity, however usually water is able to conduct electricity because of the impurities it contains.
If you add salt to pure water, the water will be able to carry electricity and you will get a shock (and probably die).




